- Anti-Aging
- Anti-Oxidant
- Pro-Hormone
- Vitamins
- Minerals
Physician formulated to RESULT in:
-
Boosting your ENERGY
-
Increasing your alertness
-
Rejuvenating your metabolism
-
Losing Weight
These days, it seems as though most people are trying to lose weight, and for good reason: about 97 million Americans are overweight or obese.
Being over-weight causes an increased risk of all-cause mortality, as well as increased morbidity from hypertension, dyslipidemia, type 2 diabetes, coronary heart disease, stroke, gallbladder disease, osteoarthritis, sleep apnea, and other respiratory problems. In addition to certain malignancies, such as cancers of the endometrium, prostate, and breast.
The National Heart, Lung, and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Disease published guidelines for the treatment of overweight and obese adults. These recommendations are intended for patients with a body mass index (BMI) ≥ 30, or ≥ 27 with obesity-related risk factors or diseases present. The guidelines recommend initial lifestyle modifications, including a reduced-calorie diet of 500-1000 calories per day, increased physical activity, and behavioral therapy. If these changes fail to produce a result in 6 months, pharmacotherapy is recommended.
These are the prescription medications approved by the US Food and Drug Administration (FDA) for weight loss: sibutramine (Meridia, by Abbott Laboratories), which inhibits the reuptake of serotonin, norepinephrine, and dopamine; orlistat (Xenical and Alli) a reversible inhibitor of gastric and pancreatic lipase which makes you expel “wet gas and floating diarrhea”; phentermine, an adrenergic medication, diethylpropion, benzphetamine, and phendimetrazine. Off-label medications for weight loss include carbohydrate and fat binders, thyroid supplements, metformin, and newer antidepressants (fluoxetine, sertraline, bupropion) and novel anticonvulsants (topiramate, zonisamide). And if all else failed, bariatric surgery is recommended in cases of extreme obesity.
Sympathomimetic amines have been used as over-the-counter diet aids, including phenylpropanolamine, Ma Huang (ephedra), and ephedrine. These products are notorious for causing dose-related increases in blood pressure that can be hazardous. When the use of phenylpropanolamine (Acutrim, by Amerifit Brands) was correlated with hypertension and stroke, the FDA banned it from the market in November 2000. Likewise, Ma Huang and ephedrine-containing supplements have been removed from the US market.
Improve your overall medical and physical health, IT'S FOR YOUR LIFE, not your looks that matter.
Remember that looking better is easy, but it's a quick fix that won't last. Becoming and STAYING healthy requires life-long changes in your Core Behaviors: eating habits and exercise levels.
Throughout much of recorded history, people struggled to get enough food to eat. Today, many people in the world, and even in the United States, remain undernourished. However, the majority of Americans face the opposite situation: they are overweight and find it difficult to lose the unwanted pounds. This is a major public health issue because being overweight can lead to serious health problems and death.
Every year in the United States, about 300,000 adults die from causes related to excess body weight. Look at the slide on the right and focus on the orange bars. Before 1998 if you had diabetes, your risk of a first heart attack is 20%; and if you had a heart attack and are diabetic then your risk of a second MI is 50%.
Why do I need to lose weight if I am overweight?
Being overweight increases your risk for high blood pressure, heart disease, stroke, diabetes, and some forms of cancer. If you are overweight, losing just 5 to 10% of your weight and keeping it off lowers your risk for developing most of these diseases.
How can I know if I am overweight?
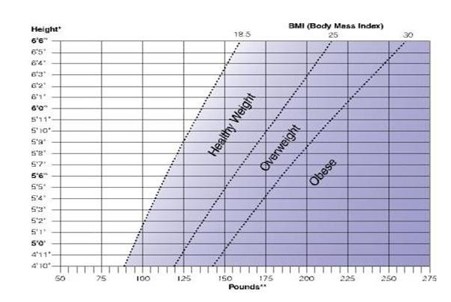
The number you see on the scale doesn't necessarily tell you whether you need to lose weight. That's because 2 people of the same height and weight can have different bone structures. They may carry different amounts of muscle and body fat. For most adults, determining your body mass index (BMI) and waist size are reliable ways to tell whether you are overweight and to estimate your risk for health problems.
Your BMI uses your height and weight to estimate how much fat is on your body. A BMI of at least 25 indicates overweight. A BMI of 30 or more indicates you are obese. Generally, the higher your BMI, the higher your weight risk. Your waist size indicates whether you have an apple shape and tend to carry fat around your midsection. Your health risks increase even further with increasing waist size. A waist measurement greater than 40 inches for men or 35 inches for women indicates a significant increase in health risk.
To tell whether your weight is a health risk, you can determine your BMI and health risk with the Body Mass Index chart:
What is Metabolic Syndrome?
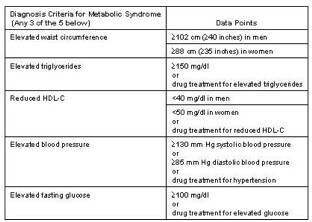
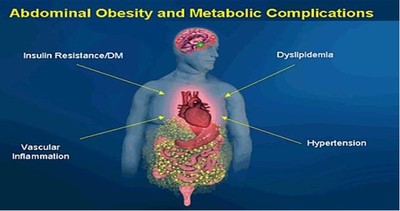
Metabolic Syndrome was first defined by an endocrinologist, Dr. Raven, who associated obesity with insulin resistance. This defining association led other physician researchers in the field of cardiology to recognize the significantly increased risk in cardiac disease in diabetic patients. Hence, Metabolic Syndrome was revealed formally in 2001 as a constellation of risk factors that accelerate atherosclerotic cardiovascular disease (ASCVD).
Cardiac Risk in Metabolic Syndrome is Greater than the Sum of its Parts
The question is constantly raised as to whether the risk for heart disease associated with the metabolic syndrome is greater than the sum of its risk factors. The answer is the YES!!
Epidemiological studies strongly show that multiple risk factors raise risk more than the sum of accompanying single risk factors; risk rises geometrically instead of linearly. This phenomenon is called multiplicative risk.
This does not include several metabolic risk factors in standard risk algorithms; which continue to increase your risk. These are a prothrombotic state, a proinflammatory state, and elevated triglyceride. This additional risk exceeds that which can be explained by standard risk factors.
Remember that because metabolic syndrome often progresses and culminates in type 2 diabetes, the syndrome's long-term risk is underestimated at any one time. Thus several lines of evidence indicate that the risk accompanying the metabolic syndrome is greater than the sum of its measured components.
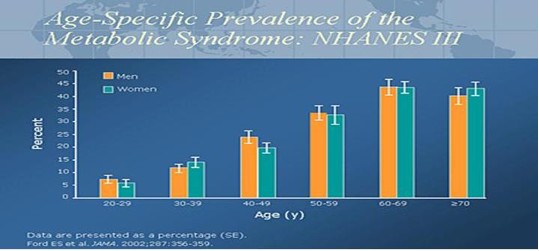
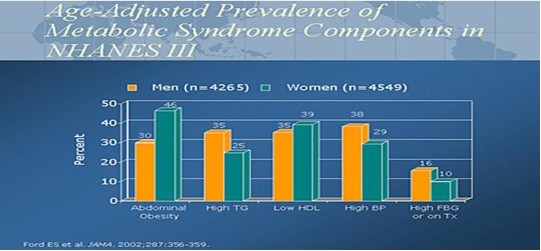
In 2002, the National Health and Nutrition Examination Survey (NHANES) data published a prevalence rate of 20% to 24% for metabolic syndrome in the US adult population. The range of Metabolic Syndrome is 5% for the age group 20-29 years old to a high of 45% for both males and females age 60 – 69 years old. The graph clearly illustrates the increase in Metabolic Syndrome with age.
Metabolic Syndrome accelerates and worsens abnormal cholesterol levels or ratios, elevated blood pressure, elevated fasting blood sugars, the prothrombotic state (clotting), and the proinflammatory state. For instance:
Cholesterol: abnormal cholesterol levels or ratios consists of elevated triglycerides, low levels of high-density lipoproteins (HDL), and increased levels of low density lipoproteins (LDL).
Glucose: Elevated fasting glucose falling in the range of either pre-diabetes or diabetes.
Clotting: A prothrombotic (clotting) state signifies anomalies in procoagulant (clot forming) factors (i.e., increases in fibrinogen and factor VII), anti-fibrinolytic (clot dissolving) factors (i.e., increases in plasminogen activator inhibitor-1), platelet aberrations, and endothelial (blood vessel)dysfunction.
Inflammation: A proinflammatory state is characterized by elevations of circulating acute and chronic phase reactant proteins (e.g., HS-CRP, ESR, etc).
This age adjusted graph demonstrates the metabolic diseases found as a percentage in the diagnosis of Metabolic Syndrome (Diabetes, Hypertension, Cholesterol abnormalities: HDL/TG, and increased abdominal girth).
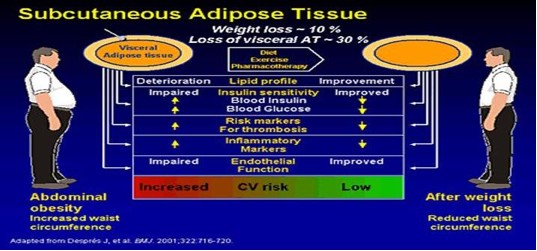
The major underlying risk factors are obesity and insulin resistance. The risk associated with obesity is best identified by increased waist circumference (visceral obesity). Insulin resistance can be secondary to obesity. The increasing prevalence of metabolic syndrome in the U.S. and worldwide, seems to be driven largely by more obesity exacerbated by sedentary lifestyles. Losing 10% of your body weight significantly IMPROVES your Metabolic Syndrome risk profile.
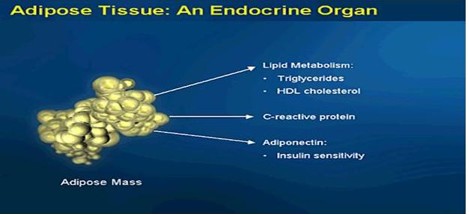
Obesity and Metabolic Syndrome
Obesity as defined by a BMI of greater than 30 when accompanied by an abdominal girth of greater than 35 for a woman or greater than 40 for a man. Visceral (abdominal) fat is an endocrine organ that secretes various proteins.
Cytokine proteins are produced by fat cell and are associated with inflammation of the heart, arteries, kidneys, muscles, and joints. Fat cell cytokines are interleukin 1 and 6, C-Reactive Protein, tumor necrosis factor alpha, adiponectin, and plasminogen activator inhibitor 1.
Losing Weight vs. Not Gaining Weight
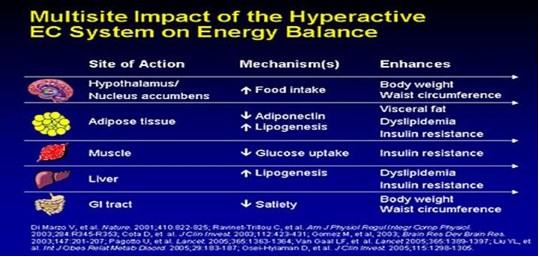
The average American gains 40 pounds between the ages of 20 and 40. Ever ask WHY. Because as you age, your neuro-endocrine system begins to slow down. This in plain english means that your thyroid, adrenal, pancreas, and testicles/ovaries glands don't work as well so you begin to feel sluggish and constantly tired, leading to decreased physical activity and weight gain. As you gain weight, your body begins to store the energy that you used to burn through exercise into FAT. Now the fat becomes another endocrine organ, releasing enzymes to make you fatter, more tired, and achy.
The more fatter, tired, and achy you become, the more gratification you receive from eating unhealthy foods. This occurs through stimulation of our “feel good” neuro-endocrine EC system. The EC system gives you a strong sense of well-being and satisfaction through eating. The more you eat, the better and more satisfied you feel. The better and more satisfied you feel, the more you want to eat.
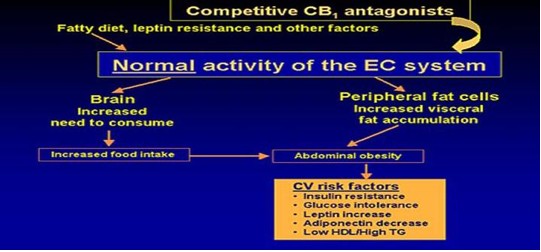
This cycle continues until you are out of control. Your intestines start to slow down and have decreased secretion of proteins to absorb essential nutrients (B12, Folate, etc) and micronutrients (zinc, chromium, etc,). Your sleep-wake cycle falters. Overall, your neuro-endocrine system becomes markedly depressed and your weight gain accelerates. It's a vicious cycle.
At the end you notice your weight is profound, your liver is fatty and enlarged, your heart is weak, your lipid levels are extremely abnormal, and your joints achy when you move.
DHEA: Dehydroepiandrosterone
DHEA and DHEAS output is maximal between the ages of 20 and 30 years and then starts a decline of approximately 2% per year, leaving a residual of 10-20% of the peak production by the eighth or ninth decade of life.
DHEA and DHEAS are interconvertible by sulfohydrolases in peripheral and adrenal tissues. Some 64-74% of the DHEAS produced each day is converted to DHEA, but only 13% of the DHEA produced is metabolized to DHEAS. In humans, the brain-to-plasma ratios for DHEA and DHEAS are 4-6.5 and 8.5, respectively, indicating a neuroendocrine role for these hormones.
DHEA and DHEAS serve as the precursors of approximately 50% of androgens in men, 75% of active estrogens in premenopausal women, and 100% of active estrogens after menopause. There appears to be a sex-specific response to DHEA replacement therapy in humans. In postmenopausal women (ages 50-65), supraphysiological doses of DHEA have predominantly androgenic effects, increasing testosterone levels approximately 300% over baseline levels.
Several mechanisms of action of DHEA and DHEAS other than their role as precursors of the sex hormones have been proposed. In the central nervous system, both DHEA and DHEAS appear to affect neurotransmitter receptors. DHEAS binds to the GABA-RC and acts as a negative noncompetitive modulator of GABA-RC. DHEA, on the other hand, has GABA-agonist effects on the GABA-RC. Also, DHEA and DHEAS have neurotrophic effects, increasing the number of neurofilament-positive neurons and regulating the motility and growth of corticothalamic projections in cultured mouse embryo brain cells.
Supraphysiological oral doses of DHEA in humans have been found to inhibit the synthesis of thromboxane A 2 in activated platelets, reduce plasma plasminogen activator inhibitor type 1 and tissue plasminogen activator antigen, increase serum levels of insulin-like growth factor 1 (IGF-1), and increase cyclic guanosine monophosphate (GMP) and nitric oxide synthesis (either directly or via increased levels of IGF-1).
These effects suggest that DHEA are beneficial in improving circulation in the microvasculature and regulating some of the risk factors of cardiovascular disease, such as platelet aggregation and ischemia. The majority of clinical studies in this area have shown an inverse relationship between DHEA or DHEAS levels and cardiovascular morbidity and mortality.
DHEA may play a positive role in modulation of the immune response. Clinical studies in elderly persons have demonstrated that oral DHEA doses of 50 mg/day increase IGF-1 levels (p < 0.01) and cause functional activation of T cells (increases in CD8+ and CD56+ cells [natural killer cells] and enhanced cytotoxic activity). Serum levels of interleukin- 6 (a proinflammatory cytokine involved in the pathogenesis of osteoporosis, rheumatoid arthritis, atherosclerosis, Alzheimer's disease, Parkinson's disease, and beta-cell malignancies) increase significantly with age and are inversely correlated with serum DHEA and DHEAS levels (p < 0.001). In addition, DHEA, DHEAS, and androstenedione inhibit the production of interleukin-6 by peripheral blood mononuclear cells in a concentration-dependent manner (p < 0.001).
Serum Dehydroepiandrosterone Sulfate Levels Predict Longevity in Men: 27-Year Follow-Up Study in a Community-Based Cohort
Objectives: To determine whether serum dehydroepiandrosterone sulfate (DHEAS) levels could predict longevity in residents.
Results: Baseline DHEAS levels were higher in men than in women and decreased with age in both sexes. In a Cox proportional hazards model, age, DHEAS (inversely), blood pressure, and fasting plasma glucose were significantly associated with shorter longevity in men but not in women. Of these variables, high DHEAS levels in men were the strongest predictor of longevity (β = -2.032, hazard ratio = 0.131, 95% confidence interval = 0.029–0.584 in the Cox proportional hazards model after adjustment for age). The Kaplan-Meier survival curve, stratified according to tertiles of DHEAS levels, in men after adjustments for age, systolic blood pressure, and fasting plasma glucose showed significantly (log-rank stat = 10.6; P <.001) greater longevity in the highest group (200 μg/dL) than in the moderate (130–199 μg/dL) or lowest groups (129 μg/dL).
Conclusion: This 27-year study in a community-based cohort indicated that DHEAS level may be a predictor of longevity in men, independent of age, blood pressure, and plasma glucose.
Effects of Low Testosterone on Future evelopment of Type 2 Diabetes and/or Metabolic Syndrome
A recent meta-analysis reported that men in the upper vs lower dichotomy of testosterone values had a 42% lower risk of developing type 2 diabetes. Included in this analysis was a large prospective Finnish study that also looked at future development of metabolic syndrome. In their report they compared men with testosterone levels in the lower quartile vs those in the upper quartile; the average follow-up was 11 years. They found that:
-
35% of men in the lowest quartile had developed metabolic syndrome alone
-
45% had developed metabolic syndrome and type 2 diabetes
-
33% had developed type 2 diabetes alone
-
20% failed to develop either metabolic syndrome or type 2 diabetes.
The possibility that low testosterone levels predict future development of the metabolic syndrome also is supported by a report from the Massachusetts Male Aging Study.
The Hypogonadism in Males (HIM) study is an industry-sponsored study that measured testosterone levels in men 45 years of age and older who presented to 130 offices of primary care clinicians over a 2-week period and who consented to participate in the study:
-
Testosterone levels < 300 ng/dL were found in 836 of 2165 men (38.6%).
-
The prevalence increased from 34% in the 45- to 54-year-old age group to 39.9% in the 65-74, 45.5% in the 75-84, and 50% in the 80+ age groups.
-
The prevalence of low total testosterone levels were found in men with obesity (52.4%), diabetes (50%), hypertension (42.4%), hyperlipidemia (40.4%), osteoporosis (44.4%).
Testosterone replacement in men with low testosterone levels can bring about a number of beneficial effects.
-
· Increased bone density
-
· Increased skeletal muscle mass and perhaps strength
-
· Decreased fat mass
-
· Improved libido and perhaps erectile function
-
· Increased red blood cell mass
-
· Improved mood
-
· Improved cognition
-
· Reduced cardiovascular events
Because men under 50 have more cardiovascular events than women in the same age group, it has been thought that testosterone may be causative, but other epidemiologic data suggest that normal testosterone levels may in fact decrease cardiovascular disease. As yet, no studies have been undertaken to evaluate the effects of testosterone replacement on cardiovascular events.
It is important to measure serum testosterone in patients receiving replacement therapy to determine whether treatment is raising the level to the desired range. The serum level required to treat organ systems varies, but in general I strive to get the levels in the 400-600 range. Some studies recommend a range of 350-500 in older men.
In the older man it is very important to monitor for potential adverse effects of testosterone treatment and for delivery system-specific adverse effects. The most common adverse effect is an excessive rise in the hematocrit (> 54%). When this occurs, I stop treatment to allow the hematocrit to normalize, after which treatment may be resumed at a lower dose.
The most serious safety concern is the potential for stimulating an occult prostate cancer to become a clinical prostate cancer. We can use the prostate cancer risk calculator (http://www.compass.fhcrc.org/edrnnci/bin/calculator/main.asp) to evaluate risk prior to initiating treatment. Should my patient develop a significant rise in hematocrit or PSA, or an abnormal DRE, it would be easier to stop treatment while further evaluation is being conducted. This usually is of less concern after a patient has been on testosterone replacement therapy for 3 months.
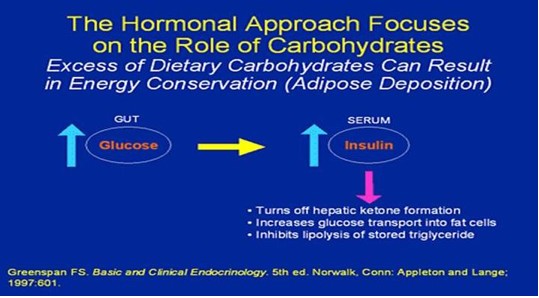
Eating better
Trends in eating habits may help explain why so many people in the United States are overweight and obese today. Americans currently consume 23% more sugar than we did in 1970, and soft drinks are the major source of the added sugar in our diets. On average, the foods we ate had 24% more total fat in 2000 than in 1970. And we eat meals away from home roughly twice as much as we used to.
DROP THE CARBS!! DROP THE FATS!!! INCREASE PROTEIN!!
Being more active
Regular physical activity has been shown to help prevent heart disease, type 2 diabetes and osteoporosis, as well as other chronic conditions. It is important for maintaining your good health, regardless of whether your weight is a problem or not.
Stress and Exercise: Getting the Balance Right for Aging Immunity
The immune response is negatively modified by both aging and chronic stress. The effects extend to both the innate and adaptive immune systems and have clinical significance in that they combine to result in reduced vaccination responses and an increased incidence and severity of infections and mortality. An altered stress reaction, as a result of adrenopause, may be a significant component of immune suppression at times of chronic stress. Several interventions have been proposed to improve immunity, and we hypothesize that regular moderate aerobic exercise may have real benefits for immunity. At times of physical stress, such as after a hip fracture or surgery, short-term pharmacological intervention with DHEA to counteract elevated stress hormones is likely to be more appropriate. We conclude that maintaining a healthy balance of stress hormones is essential to optimizing immunity.
Thank you. I hope I have provided you with enough academic information to motivate you changing your life and the lives of those you love.
May God Bless You and Your Family.
Steven Dominguez, M.D., M.P.H.
-
UCLA School of Public Health; B.S. and M.P.H.
-
University of Iowa College of Medicine, M.D.
-
Cleveland Clinic Foundation Head and Neck, Facial Plastic Surgery Program
-
UCLA Emergency Medicine Program
Now the legal stuff: The statements herein have not been evaluated by the FDA and are not intended to advise, diagnose, treat, cure, prevent, or infer medical care. Seek medical advice from your physician prior to purchasing or using any products represented in this website.
References
-
Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-3421.
-
Narayan KM, Boyle JP, Thompson TJ, et al. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884-1890.
-
American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005;28(suppl 1):S4-S36.
-
Grundy SM, Brewer HB Jr, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433-438.
-
Orchard TJ, Temprosa M, Goldberg R, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142(8):611-619.
-
Gades MD, Stern JS. Chitosan supplementation does not affect fat absorption in healthy males fed a high-fat diet, a pilot study. Int J Obes (Lond). 2002;26:119-122.
-
Mhurchu CN, Poppitt SD, McGrill AT, et al. The effect of the dietary supplement, chitosan, on body weight: a randomized controlled trial in 250 overweight and obese adults. Int J Obes (Lond). 2004;28:1149-1156.
-
Gades MD, Stern JS. Chitosan supplementation and fat absorption in men and women. J Am Diet Assoc. 2005;105:72-77.
-
Pasman WJ, Westerterp-Plantenga MS, Saris WHM. The effectiveness of long-term supplementation of carbohydrate, chromium, fibre, and caffeine on weight maintenance. Int J Obes (Lond). 1997;21:1143-1151.
-
Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149-157.
-
Charles MA, Morange P, Eschwete E, et al. Effect of weight change and metformin on fibrinolysis and the von Willebrand factor in obese non-diabetic subjects: the BIGPRO1 study. Biguanides and the Prevention of the Risk of Obesity. Diabetes Care. 1998;21(11):1967-1972.
-
Lehtovirta M, Forsen B, Gullstrom M, et al. Metabolic effects of metformin in patients with impaired glucose tolerance. Diabetes Med. 2001; 18(7):578-583.
-
Li CL, Pan CY, Lu JM, et al. Effect of metformin on patients with impaired glucose tolerance. Diabetes Med. 1999; 16(6):477-481.
-
Ramachandran A, Snehalatha C, Mary S, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49(2):289-297.
-
Nachtigal MC, Patterson RE, Stratton KL, Adams LA, Shattuck AL, White E. Dietary supplements and weight control in a middle-age population. J Alt Comp Med. 2005;11:909-915.
-
Blum K, Cull JG, Chen TJH, et al. Clinical evidence for effectiveness of PhenCal in maintaining weight loss in an open-label, controlled, 2-year study. Curr Ther Res. 1997;58:745-763.
-
Lopez-Carmona JM, Rodriguez-Moctezuma JR, Ariza-Andraca CR, et al. Lifestyle and metabolic control in patients with type 2 diabetes mellitus. Construct validation of IMEVID questionnaire [in Spanish]. Aten Primaria. 2004;33(1):20-27.
-
Hoeger KM, Kochman L, Wixom N, et al. A randomized, 48-week, placebo-controlled trial of intensive lifestyle modification and/or metformin therapy in overweight women with polycystic ovary syndrome: a pilot study. Fertil Steril. 2004;82(2):421-429.
-
Diabetes Prevention Program Research Group. Effects of withdrawal from metformin on the development of diabetes in the diabetes prevention program. Diabetes Care. 2003;26(4):977-980.
-
Johnson JA, Simpson SH, Toth EL, et al. Reduced cardiovascular morbidity and mortality associated with metformin use in subjects with type 2 diabetes. Diabetes Med. 2005;22(4):497-502.
-
Herman WH, Hoerger TJ, Brandle M, et al. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med. 2005;142(5):323-332.
-
DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999;131(4):281-303.
-
Salpeter S, Greyber E, Pasternak G, et al. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006(1):CD002967.
-
Gerstein HC, Yusuf S, Bosch J, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368(9541):1096-1105.
-
Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007; 356(24):2457-2471.
-
A more detailed discussion of this topic can be found in Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149-157.
-
Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403.
-
Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001; 344(18):1343-1350.
-
Andraws R, Brown DL. Effect of inhibition of the renin-angiotensin system on development of type 2 diabetes mellitus (meta-analysis of randomized trials). Am J Cardiol. 2007;99(7):10061012.
-
Gerstein HC, Yusuf S, Bosch J, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368(9541):1096-1105.
-
Knowler WC, Hamman RF, Edelstein SL, et al. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes. 2005; 54(4):1150-1156.
-
Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149-157.
-
Diabetes Prevention Program Research Group. Effects of withdrawal from metformin on the development of diabetes in the diabetes prevention program. Diabetes Care. 2003;26(4): 977-980.
-
UK Prospective Diabetes Study (UKPDS) Group. UK prospective diabetes study 16. Overview of 6 years' therapy of type 2 diabetes: a progressive disease. Diabetes. 1995; 44(11):1249-1258.
-
UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854-865.
-
Viberti G, Kahn SE, Greene DA, et al. A diabetes outcome progression trial (ADOPT): an international multicenter study of the comparative efficacy of rosiglitazone, glyburide, and metformin in recently diagnosed type 2 diabetes. Diabetes Care. 2002; 25(10):1737-1743.
-
Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007; 356(24):2457-2471.
-
Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559.
-
Lukaski HC, Siders WA, Penland JG. Chromium picolinate supplementation in women: effects on body weight, composition, and iron status. Nutrition. 2007;23:187-195.
-
Kaats GR, Blum K, Pullin D, Keith SC, Wood RW. A randomized, double-masked, placebo-controlled study of the effects of chromium picolinate supplementation on body composition: a replication and extension of a previous study. Curr Ther Res. 1998;59:379-388.
-
Vincent JB. The potential value and toxicity of chromium picolinate as a nutritional supplement, weight loss agent and muscle development agent. Sports Med. 2003;33:213-230.
-
Larsen TM, Toubro S, Gudmundsen O, Astrup A. Conjugated linoleic acid supplementation for 1 y does not prevent weight or body fat regain. Am J Clin Nutr. 2006;83:606-612.
-
Kamphiuis MMJW, Lejeune MPGM, Saris WHM, Westerterp-Plantenga MS. Effect of conjugated linoleic acid supplementation after weight loss on appetite and food intake in overweight subjects. Eur J Clin Nutr. 2003;57:1268-1274.
-
Howarth NC, Saltzman E, McCrory MA, et al. Fermentable and nonfermentable fiber supplements did not alter hunger, satiety or body weight in a pilot study of men and women consuming self-selected diets. J Nutr. 2003;3141-3144.
-
Rigaud D, Ryttig KR, Angel LA, Apfelbaum M. Overweight treated with energy restriction and a dietary fibre supplement: a 6-month randomized, double-blind, placebo-controlled trial. Int J Obes (Lond). 1990;14:763-769.
-
Rossner S, von Zweigbergk D, Ohlin A, Ryttig K. Weight reduction with dietary fibre supplements. Results of two double-blind randomized studies. Acta Med Scand. 1987;222:83-88.
-
Kovacs EM, Lejeune MP, Nijs I, Westerterp-Plantenga MS. Effects of green tea on weight maintenance after body-weight loss. Br J Nutr. 2004;91:431-437.
-
Chan CCW, Koo MWL, Ng EHY, Tang OS, Yeung WSB, Ho PC. Effects of Chinese green tea on weight, and hormonal and biochemical profiles in obese patients with polycystic ovary syndrome -- a randomized placebo-controlled trial. J Soc Gynecol Invest. 2006;13:63-68.
-
Mollinari M, Watt KDS, Kruszyna T, et al. Acute liver failure induced by green tea extracts: case report and review of the literature. Liver Transplant. 2006;12:1892-1895.
-
Pittler MH, Schmidt K, Ernst, E. Adverse events of herbal food supplements for body weight reduction: systematic review. Int Assoc Study Obesity. 2005;6:93-111.
-
Heini AF, Lara-Castro C, Schneider H, Kirk KA, Considine RV, Weinsier RL. Effect of hydrolyzed guar fiber on fasting and postprandial satiety and satiety hormones: a double-blind, placebo-controlled trial during controlled weight loss. Int J Obes (Lond).1998;22:906-909.
-
Anderon T, Fogh J. Weight loss and delayed gastric emptying following a South American herbal preparation in overweight patients. J Hum Nutr Diet. 2001;14:243-250.
-
MacLean DB, Luo LG. Increased ATP content/production in the hypothalamus may be a signal for energy-sensing of satiety: studies of the anorectic mechanism of a plant steroidal glycoside. Brain Res. 2004;1020:1-11.
-
Phytopharm. Hoodia factfile. Phytopharm 2006. Available at: http://www.phytopharm.com/hoodiafactfile/ Accessed February 28, 2008.
-
Heymsfield SB, Allison DB, Vasselli JR, Pietrobelli A, Greenfield D, Nuneq C. Garcinia cambogia (hydroxycitric acid) as a potential antiobesity agent. JAMA. 1998;280:1596-1600.
-
Kriketos AD, Thompson HR, Greene H, Hill JO. (-)-Hydroxycitric acid does not affect energy expenditure and substrate oxidation in adult males in a post-absorptive state. Int J Obes (Lond). 1999;24:867-873.
-
Westerterp-Plantenga MS, Kovacs EMR. The effect of (-)-hydroxycitrate on energy intake and satiety in overweight humans. Int J Obesity. 2002;26:870-872.
-
Udani J, Hardy M, Madsen DC. Blocking carbohydrate absorption and weight loss: a clinical trial using Phase 2 brand proprietary fractionated white bean extract. Alternative Med Rev. 2004;9:63-69.
-
Celleno L, Tolaini MV, D'Amore A, Perricone NV, Preuss HG. A dietary supplement containing standardized Phaseolus vulgaris extract influences bodyd composition of overweight men and women. Int J Med Sci. 2007;4:45-52.
-
Boullata JI, MDonnell PJ, Oliva CD. Anaphylactic reaction to a dietary supplement containing willow bark. Ann Pharmacother. 2003;37:832-835.
-
Flegal KM, Carroll MD, Kuczmarski RJ et al. Overweight and obesity in the United States: prevalence and trends, 1960-1994. Int J Obes. 1998; 22:39-47.
-
Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med. 1993; 119(7, pt. 2):655-60.
-
McGinnis M, Foege WH. Actual causes of death in the United States. JAMA. 1993; 270:2207-12.
-
Wolf AM, Colditz GA. Social and economic effects of body weight in the United States. Am J Clin Nutr. 1996; 63(suppl):466s-9s.
-
Fontaine KR, Cheskin LJ, Barosky I. Health-related quality of life in obese persons seeking treatment. J Fam Pract. 1996; 43:265-70.
-
Healthy people 2010. Conference edition. Vols. 1 and 2. Washington, DC: U.S. Department of Health and Human Services; 2000.
-
Rosenbaum M, Leibel RL, Hirsch J. Obesity. N Engl J Med. 1997; 337:396- 407.
-
Atkinson RL. A 33-year-old woman with morbid obesity. JAMA. 2000; 283: 3236-43.
-
Williamson DF, Pamuk E, Thun M et al. Prospective study of intentional weight loss and mortality in never-smoking overweight US white women aged 40-64 years. Am J Epidemiol. 1995; 141:1128-41.
-
Bray GA. The new era of drug treatment. Pharmacologic treatment of obesity: symposium overview. Obes Res. 1995; 3(suppl 4):415s-7s.
-
Bray GA. Use and abuse of appetite- suppressant drugs in the treatment of obesity. Ann Intern Med. 1993; 119(7, pt. 2):707-13.
-
National Task Force on the Prevention and Treatment of Obesity. Long-term pharmacotherapy in the management of obesity. JAMA. 1996; 276:1907-15.
-
McNeely W, Goa KL. Sibutramine. A review of its contribution to the management of obesity. Drugs. 1998; 56: 1093-124.
-
McNeely W, Benfield P. Orlistat. Drugs. 1998; 56:241-9.
-
Mertens IL, Van Gaal LF. Promising new approaches to the management of obesity. Drugs. 2000; 60:1-9.
-
Peter CJ, Early JJ, Gibbs CM. Treatment of affective disorder and obesity with topiramate. Ann Pharmacother. 2000; 43:1262-5.
-
Dursun SM. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry. 2000; 45:198. Letter.
-
Voelker R. New weight loss tool. JAMA. 1999; 281: 2277.
-
Munro JF, MacCuish AC, Wilson EM et al. Comparison of continuous and intermittent anorectic therapy in obesity. BMJ. 1968; 1:352-4.
-
Weintraub M, Sundaresan PR, Madan M et al. Long-term weight control studies I-VII. Clin Pharmacol Ther. 1992; 51:586-641.
-
Guy-Grand B, Apfelbaum M, Crepaldi G et al. International trial of long-term dexfenfluramine in obesity. Lancet. 1989; 2:1142-5.
-
Bray GA, Blackburn GL, Ferguson JM et al. Sibutramine produces dose-related weight loss. Obes Res. 1999; 7:189- 98.
-
Jones SP, Smith IG, Kelly F et al. Long term weight loss with sibutramine. Int J Obes. 1995; 19(suppl 2):41. Abstract.
-
Apfelbaum M, Vague P, Ziegler O et al. Long-term maintenance of weight loss after a very-low-calorie diet: a randomized blinded trial of the efficacy and tolerability of sibutramine. Am J Med. 1999; 106:179-84.
-
Sjostrom L, Rissanen A, Andersen T et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. Lancet. 1998; 352:167-73.
-
Davidson MH, Hauptman J, DiGirolamo M et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat. JAMA. 1999; 281:235-42.
-
Meridia product monograph. Mount Olive, NJ: Knoll Pharmaceutical; 1998 Nov.
-
Xenical package insert. Nutley, NJ: Hoffmann-La Roche; 1999 May.
-
Connolly HM, Crary JL, McGoon MD et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997; 337:581-8.
-
Gardin JM, Schumacher D, Constantine G et al. Valvular abnormalities and cardiovascular status following exposure to dexfenfluramine or phentermine/ fenfluramine. JAMA. 2000; 283:1703-9.
-
Centers for Disease Control and Prevention. Cardiac valvulopathy associated with exposure to fenfluramine or dexfenfluramine: US Department of Health and Human Services interim public health recommendations. MMWR Morb Mortal Wkly Rep. Nov 1997; 46:1061-6.
-
Bach DS, Rissanen AM, Mendel CM et al. Absence of cardiac valve dysfunction in obese patients treated with sibutramine. Obes Res. 1999; 7:363-9.
-
Abenhaim L, Moride Y, Brenot F et al. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. N Engl J Med. 1996; 335:609-16.
-
McCann UD, Seiden LS, Rubin LJ et al. Brain serotonin neurotoxicity and primary pulmonary hypertension from fenfluramine and dexfenfluramine. JAMA. 1997; 278:666-72.
-
Xenical capsules Hoffmann-La Roche applicant no. 20-766. Approval date Apr 23, 1999. www.fda.gov/cder/foi/ nda/99/020766a.htm (accessed 2001 Apr 3).
-
Ionamin package insert. Rochester, NY: Medeva Pharmaceuticals; 1996 Aug.
-
Ulus IH, Maher TJ, Wurtman RJ. Characterization of phentermine and related compounds as monoamine oxidase (MAO) inhibitors. Biochem Pharmacol. 2000; 59:1611-21.
-
Sporer KA. The serotonin syndrome: implicated drugs, pathophysiology, and management. Drug Saf. 1995; 13: 94-104.
-
Pittler MH, Abbot NC, Harkness EF et al. Randomized, double-blind trial of chitosan for body weight reduction. Eur J Clin Nutr. 1999; 53:379-81.
-
Heymsfield SB, Allison DB, Vasselli JR et al. Garcinia cambogia (hydroxycitric acid) as a potential antiobesity agent. JAMA. 1998; 280:1596-1600.
-
Kernan WN, Viscoli CM, Brass LM et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Eng J Med. 2000; 343:1826-32.
-
Centers for Disease Control and Prevention. Adverse events associated with ephedrine-containing products -- Texas, December 1993-September 1995. MMWR Morb Mortal Wkly Rep. 1996; 45:689-92.
-
Food and Drug Administration. FDA warns against drug promotion of "herbal fenphen." Rockville, MD: U.S. Department of Health and Human Services; 1997 Nov 6. (Talk Paper T97-56.)
-
Powell T, Hsu FF, Turk J et al. Mahuang strikes again: ephedrine nephrolithiasis. Am J Kidney Dis. 1998; 32: 153-9.
-
Nadir A, Agrawal S, King PD et al. Acute hepatitis associated with the use of a Chinese herbal product, mahuang. Am J Gastroenterol. 1996; 91:1436-8.
-
Larrey D, Vial T, Pauwels A et al. Hepatitis after germander (Teucrium chamaedrys) administration: another instance of herbal medicine hepatotoxicity. Ann Intern Med. 1992; 117:129-32.
-
Ernst E. Second thought about safety of St. John's wort. Lancet. 1999; 354: 2014-6.
-
Fugh-Berman A. Herb-drug interactions. Lancet. 2000; 355:134-8.
-
Yue QY, Berquist C, Gerden B. Safety of St. John's wort (Hypericum perforatum). Lancet. 2000; 355:576-7. Letter.
-
Gurley BJ, Gardner SF, Hubbard MA. Content versus label claims in ephedracontaining dietary supplements. Am J Health-Syst Pharm. 2000; 57:963-9
-
Miller LG. Herbal medicinals. Arch Intern Med. 1998; 158:2200-11.
-
Winslow LC, Kroll DJ. Herbs as medicines. Arch Intern Med. 1998; 158: 2192-9.
-
Dombrowski SR. Pharmacist counseling on nutrition and physical activity -- part 1 of 2: understanding current guidelines. J Am Pharm Assoc. 1999; 39:479-91.
-
Dombrowski SR, Ferro LA. Pharmacist counseling on nutrition and physical activity -- part 2 of 2: helping patients make changes. J Am Pharm Assoc. 1999; 39:613-27.
-
Williams RA, Foulsham BM. Weight reduction in osteoarthritis using phentermine. Practitioner. 1981; 225:231-2.
-
Hollander PA, Elbein SC, Hirsch IB et al. Role of orlistat in the treatment of obese patients with type 2 diabetes: a 1-year randomized double-blind study. Diabetes Care. 1998; 21:1288- 94.